Designed to be minimally invasive and replaceable, NuPulse Voyage is currently implanted in the operating room and will eventually be implanted in a hybrid operating room suite or catheterization lab.

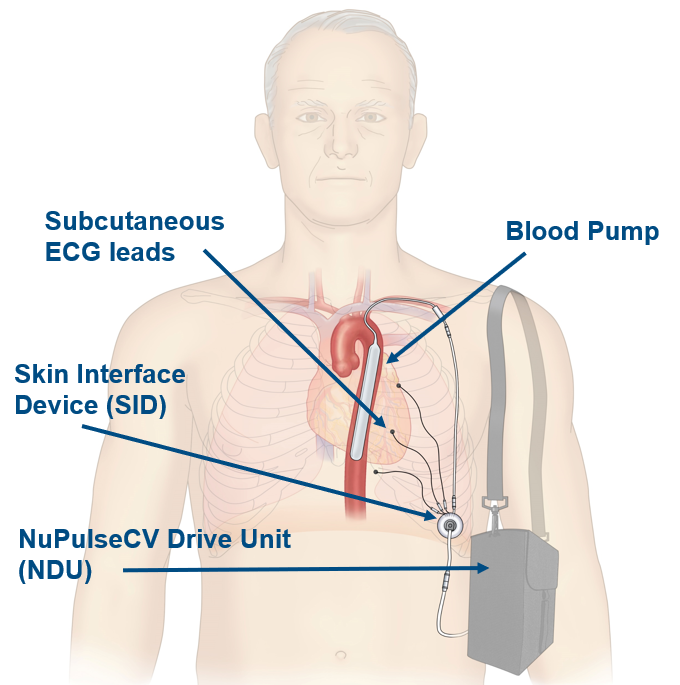
The NuPulse Voyage Device
NuPulse Voyage is the first minimally invasive, long-term, ambulatory counterpulsation device that works in sync with the heart.

Not Currently Enrolling
The NuPulse Voyage Feasibility Trial is not currently enrolling. If you are interested in learning more please visit clinicaltrials.gov.
Caution- Investigational Device, Limited by United States Law to Investigational Use
Not Available for Sale.